- How organic chemistry began and how it got its name.
- The reason that carbon's chemistry is studied much more than the chemistry of other elements.
- The basic rules of naming simple organic compounds.
- The different ways to draw and represent organic compounds and their features.
Notes:
- Organic chemistry is all about carbon compounds. Any compound that contains carbon atoms can be called an organic compound, but it usually means compounds with a carbon chain of some type.
The earliest organic chemistry started with chemicals found from living organisms like trees, other plants and animals.
Some of the most important molecules in living organisms are carbon compounds:- Amino acids that combine to make the proteins in our body.
- Nucleotides that combine to make the DNA in our cells.
- Glucose that breaks down in respiration, producing the energy for all living processes
- Carbon atoms can make four separate bonds with any other atom(s), including other carbon atoms. This allows for an incredible variety of bonding to different atoms in compounds of different sizes. Thats why there are millions of carbon compounds and organic chemistry is such a large field.
- Since there are so many different carbon compounds, chemists organize compounds by their functional group, the most reactive part or group of atoms in the molecule. We will learn specific functional groups in the next few lessons.
- Because carbon can bond to itself in these long chains, these functional group families of carbon compounds are normally then arranged in a homologous series. A homologous series is a family of compounds with the same functional group and general formula that differs from the next by -CH2-, which is a unit of a carbon chain.
- Aside from living organisms, organic molecules have a wide range of uses in our day to day lives, with most being products of crude oil and the petroleum industry. Some organic chemicals from crude oil are:
- Gasoline/petrol, fuel for cars.
- Kerosene, fuel for aircraft.
- Refinery gases used as fuels, heating homes.
- Bitumen, used to tar roads.
- 'Naphtha' which is a mix of organic compounds that are used to make many different chemicals like plastics and cosmetics.
- Atorvastatin, heart disease medication.
- Humira, a medication for many autoimmune (the body attacking itself) diseases.
- Prozac, an antidepressant.
- Salbutamol, anti-asthma medication.
- There are so many organic compounds that they are divided into categories depending on what atoms or types of bonds they contain. Amongst the simplest and most important, compounds containing only carbon and hydrogen are called hydrocarbons.
- There is a systematic way of naming organic compounds. The two most important, basic rules are below:
- Identify the longest carbon chain in the compound. This is the core of the compound name. Depending on the carbon chain length, the following names are assigned. The table below summarizes them.
- Identify the functional group(s) in the compound. This will give you the suffix (end part) of the name. A few basic examples (which we'll study in detail later) are in the table below.
- Combining these two parts will give you the name of unbranched organic compounds:
- e.g. hexane (6 carbon chain, alkane),
- butene (4 carbon chain, alkene),
- ethyne (2 carbon chain, alkyne).
- Ethanol (2-carbon alkane chain, alcohol).
- When studying organic compounds, there are many ways to represent a chemical compound that we are talking about. Some are simpler than others, and some are more appropriate in certain situations.
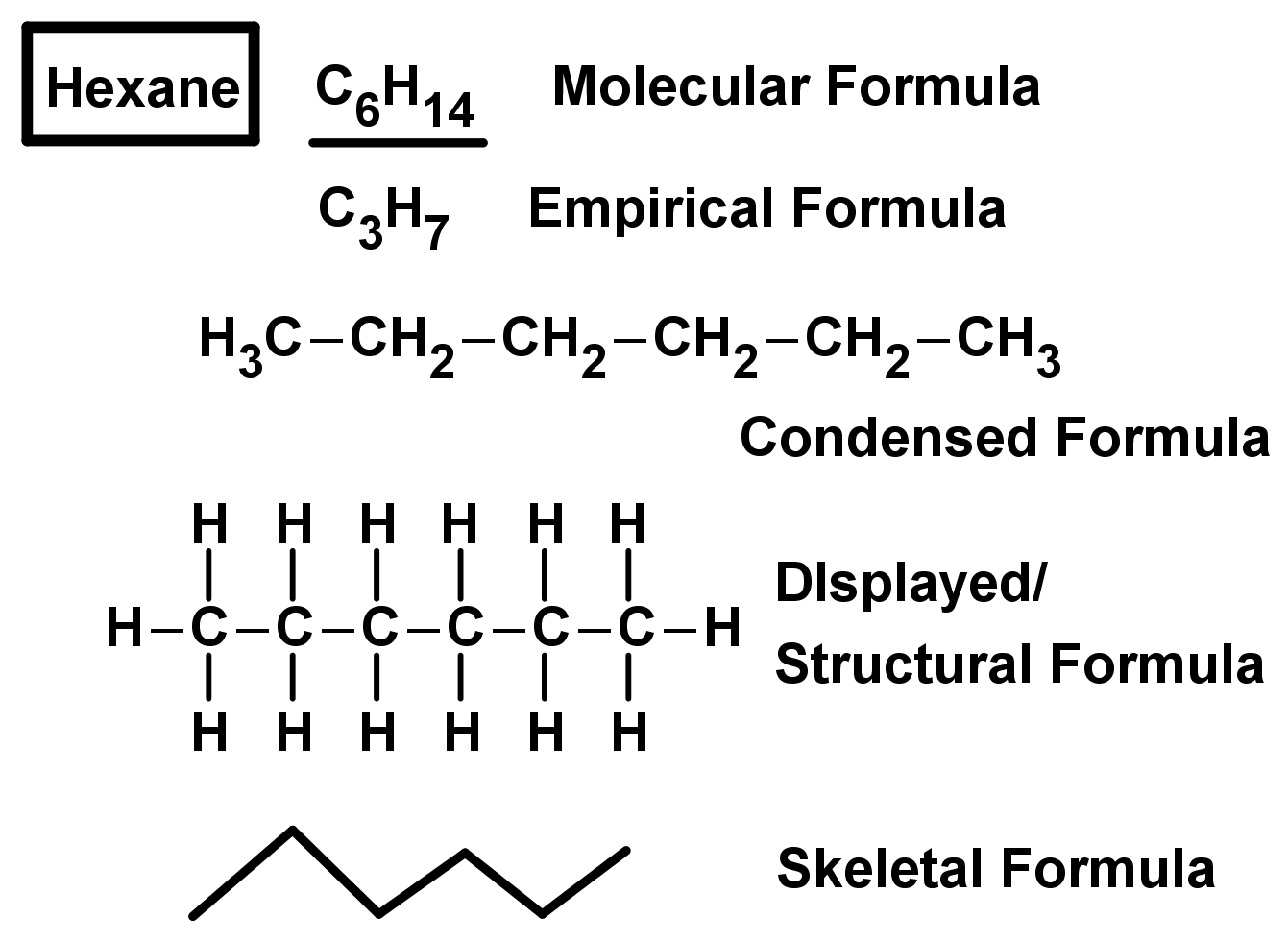
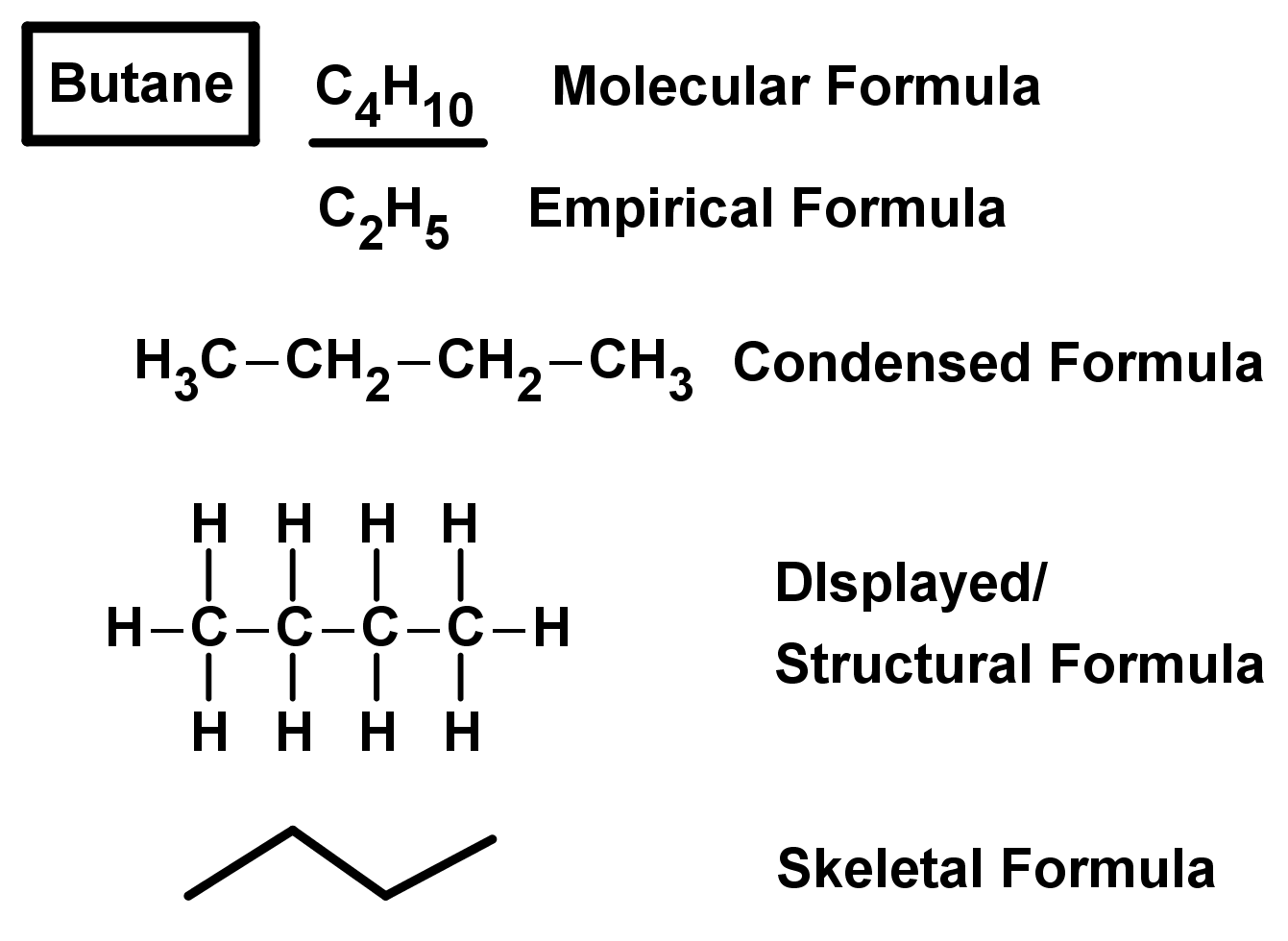
- We already know the molecular formula, which shows the chemical symbols of the atoms in the molecule and how many of these atoms there are.
- For example CH4 is a compound which has one carbon atom (symbol C) and four hydrogen atoms (symbol H) in each molecule.
- The empirical formula is a shortened version of the molecular formula which shows the smallest whole-number ratio of the molecular formula.
- For C2H6, the empirical formula would be CH3.
- The molecular formula C4H10 has the empirical formula C2H5. It cannot be CH2.5 because there is a non-integer number of atoms (you cant have half an atom in a molecule!)
- For organic compounds we can also use the condensed formula – this is used to show the chain of carbon atoms in the compound.
- For example C5H12 can be written as CH3-CH2-CH2-CH2-CH3 to show the 5 carbon chain in the molecule.
- You could also use the structural/displayed formula, which shows all the bonds between all atoms in the molecule.
- For larger organic molecules, the structural formula can be simplified by using skeletal formula to save time while keeping all the relevant information. The skeletal formula shows carbon chains as zig-zags, where the carbon atoms are the joints in the chain. In skeletal formula, hydrogen atoms are implicit meaning they not shown in the representation, but they are there. The ends of the zig zag chains count as carbon atoms as well. This means you can draw long, complicated, branching carbon chains easily with just a few zig-zag lines. We will build on skeletal formula and drawing molecules later on.
- Two examples of compounds shown with each of these formulae are shown below:
The pharmaceutical industry uses organic compounds taken from crude oil and makes medicinal organic compounds that treat many conditions, such as:
To a chemist, calling a chemical natural or artificial is meaningless. Is a treehouse unnatural? We learn nothing by saying natural or unnatural because it does not tell us a chemicals properties. There are toxic natural substances and safe, beneficial ones. The same goes with artificial substances.
|
Carbon chain length: |
Root name: |
|
1 |
Meth- |
|
2 |
Eth- |
|
3 |
Prop- |
|
4 |
But- |
|
5 |
Pent- |
|
6 |
Hex- |
|
7 |
Hept- |
|
8 |
Oct- |
|
Functional group |
Suffix |
|
Alkane |
-ane |
|
Alkene |
-ene |
|
Alkyne |
-yne |
|
Alcohol |
-ol |






