In this lesson, we will learn:
- To describe the features of metallic bonding and the structure of pure metals.
- How to explain the properties of metals using metallic bonding theory.
- How to explain the trends in properties of metals using metallic bonding theory.
Notes:
- We saw earlier that ionic and covalent bonding are bonding types that hold compounds and small molecules together, but in elemental metal samples (pure metals, not metal compounds) there is a third type of bonding called metallic bonding.
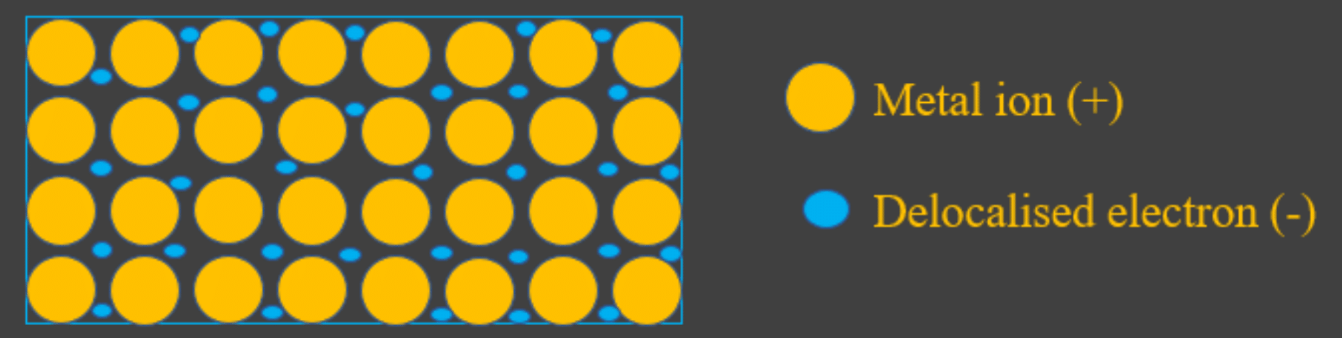
- In elemental metals (that means pure iron metal, not iron compounds), metallic bonding creates a structure with the following features:
- There is a lattice of positively charged metal ions.
- Between these positive ions, there is a sea of negative delocalised electrons. These are the electrons that the metal atoms (that are now ions!) had lost, so they could gain a full outer shell.
- Metallic bonding, occurs in samples of metal-only atoms, including pure metallic samples, and explains the properties of pure metals that we observe.
- Metallic bonding occurs in PURE METAL SAMPLES. It occurs in alloys too, which are mixtures of different metals we will look at next lesson.
- Metallic bonding does NOT occur in metal compounds with non-metal atoms. That is ionic bonding which we learned in C11.4.2: Ionic and covalent bonding
- As with any bonding theory, we use our ideas of metallic bonding to help explain the properties that we see when we study metals. Metallic bonding explains the properties of metals in the following ways:
- There is a strong electrostatic attractive force between the metal ions and the delocalised electrons. It takes a lot of energy to overcome this force and pull the positive ions apart from the delocalised electrons. This is why most metals have a high melting point.
- The sea of delocalised electrons is fluid. This means the metal ions can move amongst and around each other because they arent rigidly stuck in one place in the lattice. This is why many pure metals are both malleable and ductile:
- A material that is malleable can be bent and re-shaped when it is heated up. This is how blacksmithing works (how swords and iron tools are made), as hot metal is hammered into different shapes before it hardens as it cools.
- A material that is ductile can be bent and drawn into thin wires. Copper is very ductile and most electrical wires are made from it.
- The fluid sea of negatively charged delocalised electrons easily carry electric charge and heat energy throughout the lattice.
- This explains why metals are good conductors of both electricity and heat.
- There are trends in properties of metals, like their melting point. The trends are caused by different metallic bonding strength which is caused by two main factors:
- The charge of the metal ion in the lattice. For example, compare group 1 metals that have a 1+ ion charge and group 2 metals with ions of 2+ charge. If you compare a group 1 and group 2 metal in a period like Na and Mg, the group 2 metal will have a higher melting point because of the greater charge difference.
In short, 2+ attracting 2- is a stronger force than 1+ attracting 1-. - The ionic radius of the ion. In Periodic trends: Atomic radius, we saw that the ionic radius gets larger going down a group in the periodic table.
This means the nucleus (where the positive charge is) is further away from the delocalised electrons it is attracted to, so a larger ionic radius makes a weaker metallic bond. You can see this in the melting and boiling points of metal elements decreasing down the group column. - Metallic structure and bonding is not only seen in pure metals, but also in mixtures of different metals combined ‐ these are called alloys. Alloys are made to obtain unique or more precise properties of two or more metal elements. We will look at alloys in our next lesson, Alloys.
See the diagram below but remember that the electrons are freely moving, thats why they look a bit disorganised.