- The definition of an isotope and their difference in properties.
- The definition of relative atomic mass and relative isotopic mass.
- How isotopes come to affect the relative atomic mass of an element.
- How to calculate relative mass of samples using relative abundance.
- The method used to detect different isotopes and some of their uses.
Notes:
- So far, we have ignored the fact that many elements in the Periodic Table have decimal numbers in their relative atomic mass. If atomic mass is a measure of the number of protons and neutrons in an atom, how is it possible to have atomic mass that isn't a whole number? Remember, you cannot have half a proton or half a neutron in an atom!
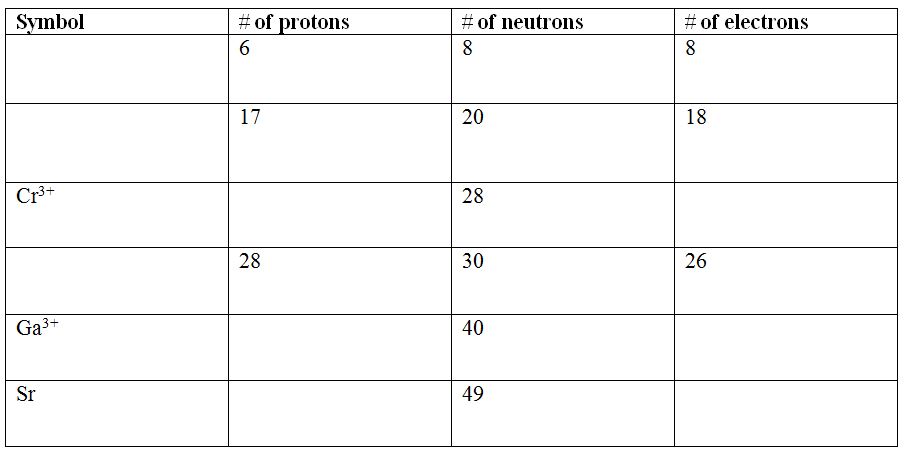
- An isotope is an atom of an element with the same number of protons but a different number of neutrons. This gives an equal proton number (so by definition it's the same element) but a different mass number. The discovery of isotopes refuted Dalton’s claim of atoms that all atoms of an element were identical – this is not true!
- Isotopes are why the periodic table contains decimals for many elements’ relative masses. Relative atomic mass for an element is an average value account for the masses and relative abundance of each isotope of an element.
- “Relative” when talking about the mass of any atom or molecule, means relative to an atom of carbon-12. The mass of any isotope or atomic sample is defined as compared to the carbon-12 (12C) isotope:
- The relative isotopic mass is the mass of an isotope relative to 1/12 of the mass of a 12C atom.
- The relative atomic mass is the mass of any atomic sample relative to 1/12 of the mass of a 12C atom.
- Any given element (defined by the proton number!) might have atoms with different numbers of neutrons. This element's range of atoms with different numbers of neutrons in them are its' isotopes.
- For example, hydrogen atoms have only 1 proton, and can only have one proton.
- Hydrogen atoms with zero neutrons are called Hydrogen-1. This is by far the most common isotope of hydrogen we observe. About 99.98% of hydrogen atoms are hydrogen-1.
- Hydrogen atoms with one neutron are called Hydrogen-2 or deuterium. This only makes up about 0.02% of any sample of hydrogen atoms.
- Another example: Carbon atoms have 6 protons in their nucleus and can only have 6 protons.
- The most common isotope of carbon atoms is carbon-12, which has 6 protons and 6 neutrons in the nucleus. Around 98.9% of carbon atoms in any sample are carbon-12.
- Carbon-13 is an isotope of carbon where the carbon atoms have 6 protons and 7 neutrons in the nucleus.
- Isotopes are normally specified by giving their relative atomic mass: e.g. carbon-13, or hydrogen-2.
- Because neutrons have no charge, the number of neutrons doesn't change an atom's chemical reactivity. Therefore isotopes of an element have identical chemical properties to each other isotope!
- Because neutrons have a relative atomic mass of 1 amu (the same as protons), isotopes do affect the relative atomic mass of elements as they are written in the periodic table. Ice cubes made of normal water (H2O) are less dense than liquid water. Ice cubes made with deuterated water (D2O), where the hydrogen atoms are hydrogen-2 atoms, sink in regular liquid water!
- It is possible to calculate molar mass of an elemental sample when given relative abundance of each isotope and individual masses. You can use the formula:
- Mn is the relative isotopic mass of the nth isotope.
- RAn is the relative abundance of the nth isotope, expressed as a decimal (for example 50% abundance is 0.5).
- (IB) Mass spectrometry is very useful for identifying different isotopes in elemental samples. Because different isotopes (e.g. 12C and 13C) have identical chemical properties, you can’t separate or identify different isotopes using chemical reactions – they will react the same way!
A mass spectrum shows the mass-to-charge ratio (m/z, effectively the mass) of atoms or molecules run through it. Since even a milligram of a substance contains billions of individual molecules or atoms, it can paint an accurate picture of the relative abundance of elemental atoms if an elemental substance was run through it. - An example of this is chlorine. In a mass spectrometer, chlorine shows clear signals at 35 and 37 m/z in a ratio of 3:1 abundance. This means that a given sample of chlorine will contain around 75% 35Cl atoms and 25% 37Cl atoms. Using the relative abundance calculation, this works out as 35.5, which is the atomic mass of chlorine as shown in the periodic table.
- (IB) There are many examples of isotopes of an element that are unstable. Eventually, they will physically break down and release some form of radiation, becoming a different chemical species in the process. This time frame of them breaking down (measured by their half-life) varies by isotope; it can be a few minutes or thousands of years. Unstable isotopes that break down and release radiation are called radioisotopes.
These are valuable as ‘tracer’ molecules where radioisotopes act as chemical ‘labels’ because we can measure the radiation released or the amount of radioisotope remaining. This has several real-world uses: - How much radioisotope remaining, compared to stable isotopes, could reveal the age of a sample. This is the basis of carbon dating. Living organisms continually ‘replace’ carbon, including the radioisotope 14C, during respiration and photosynthesis. Dead organisms do not, so levels of 14C start decreasing as it decays away, compared to the stable 12C.
In this way, the ratio of 14C:12C found in a mass spectrum can estimate a sample’s age. - How much radiation is (not) detected can show us accumulation of a substance, for example in part of a human body. This is useful in nuclear medicine: cancer cells generally have a higher metabolic rate than regular cells, so glucose and oxygen are more concentrated in them. Fluorodeoxyglucose (FDG) is very similar in structure to glucose but it contains the radioisotope 18F. Because of the higher metabolism of cancer cells, FDG accumulates in cancer cells noticeably more than regular cells. This is observed in the radiation given off by 18F in positron emission tomography (PET).
Where:
An example calculation for this is with boron. Boron occurs as two stable isotopes: 10B which has 19.9% abundance and 11B which has 80.1% abundance. The calculation for the relative atomic mass of boron using these figures would be:
Rearranging this equation, an isotope's relative abundance can be found if the relative mass is known and the other isotope abundances are too.