- What chemical symbols and formulae are, and how to read them correctly.
- Why they are used in chemistry and their advantage.
- How they are used to describe more complex chemicals.
- To understand the difference between compounds and mixtures in terms of composition.
Notes:
- A chemical symbol is a one or two letter symbol used to refer to any element in the Periodic Table. They aren’t just used as shortcuts for elements; different symbols can be combined to show the chemical formulae of compounds and molecules.
This demonstrates the particulate nature of matter. All matter in the universe is made up of extremely small particles called atoms and as we saw in Introduction: The Periodic Table, the periodic table is just a ‘list’ of all the different types of atoms (elements) we know about. These are what the chemical symbols represent. Compounds and molecules are combinations of these atoms which join up in fixed whole-number ratios to make new substances.
When this happens, the new compounds and molecules have completely different properties to their constituent atoms - the atoms they’re made of. - Table salt is a compound called sodium chloride (formula NaCl). It is perfectly safe to enjoy with food, even though sodium (Na) reacts violently with water and chlorine (Cl) is a toxic gas once used as a chemical weapon. Sodium chloride is perfectly safe because compounds have a completely different set of properties to its constituent atoms.
- Chemical formulae describe molecules, which are substances made of more than one atom combined, and compounds are chemicals made of more than one element combined. Know the difference!
- Every single chemical substance in existence is made of one or more atoms. Not all chemicals are made of one of more types of atoms! For example, the compound carbon dioxide (CO2) and the molecule nitrogen gas (N2):
We can call the individual carbon dioxide ‘pieces’ (one carbon atom and two oxygen atoms each) molecules. Carbon dioxide is a compound and a sample of it will contain millions of carbon dioxide molecules.
But nitrogen gas is not a compound because it is made of only one type of atom - nitrogen. It is a chemical made of two nitrogen atoms combining to form a molecule, not a compound. - Chemical formulae for chemical compounds obey Proust's law, or the law of definite proportions: A chemical formula shows the ratio of each element (measured by mass) in a chemical compound. This ratio is always true regardless of how the chemical was prepared.
The advantage of chemical formulae over written names of chemicals is that they can specify the exact number of each atom in that chemical, as a ratio of all the elements it's made of.- For example: magnesium hydroxide, Mg(OH) and sodium hydroxide, NaOH. Here, the chemical formulae clarifies the 'hydroxide' term as the first compound has two hydroxide groups, and the second has only one.
- Be careful with capital letters in chemical formulae. The second letter of chemical symbols are always lower case, so a capital letter always shows a new element symbol.
- For example: CO and Co are two completely different chemicals. CO is a compound of carbon (C) and oxygen (O) called carbon monoxide, whereas Co is symbol for the metal element Cobalt.
- Numbers in subscript are used to specify how many of this atom are present in a molecule of the chemical. Do not confuse this with {superscript which is not used in chemical formulae. For example: Na2O is a compound that is made of two atoms of sodium and one atom of oxygen.
- Brackets ( ) followed by numbers in chemical formulae are used to show that every atom contained in the brackets is present in that quantity. his is like in math where the whole bracket is multiplied by the number beside it.
- For example: the chemical formula Mg(OH)2 shows that, as a ratio, this compound contains two of both O and H atoms for each Mg atom.
- If there is no number in subscript written after a chemical formula, it means that only one of that type of atom is present in this chemical.
- When writing simpler chemical formulae, the metal (and hydrogen) atoms are normally written first, followed by non-metal atoms or groups of atoms in a formulae.
- Do not confuse compounds with mixtures. There is a big difference between the two in terms of their composition:
- A compound is entirely made up of molecules with only one chemical identity – the molecule that we call a compound, which is made of more than one element.
For example, imagine a bathtub of pure water. This is a compound because the entire substance is made up of H2O molecules (a lot of them!).
Because the entire substance is made of the same H2O chemical species, we can expect that any small sample of it will have the same properties and make-up of the rest of it; it’s a uniform single-phase substance. We call these homogeneous substances.
See below for an image of water as an example of a compound and a homogeneous substance – the entire substance is made of one type of chemical: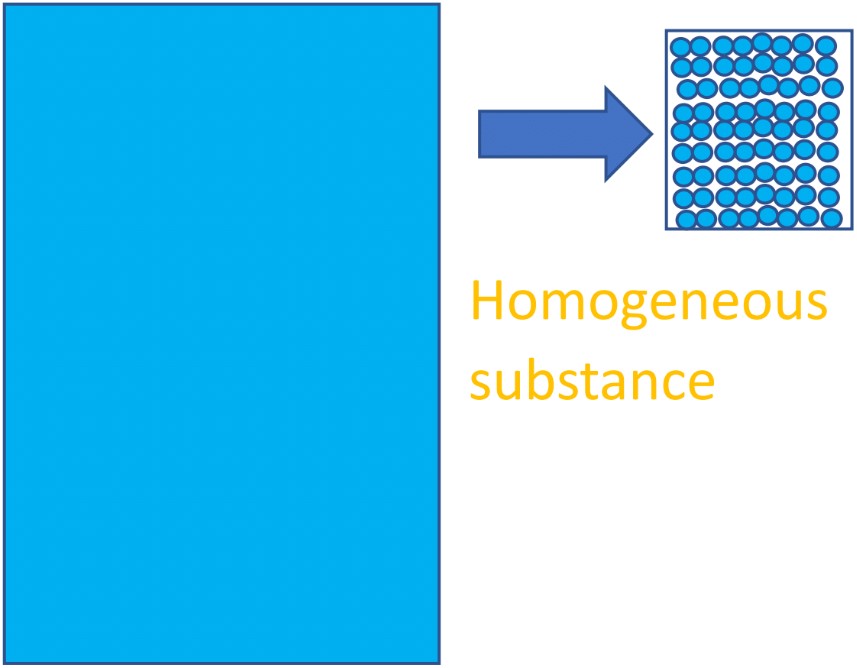
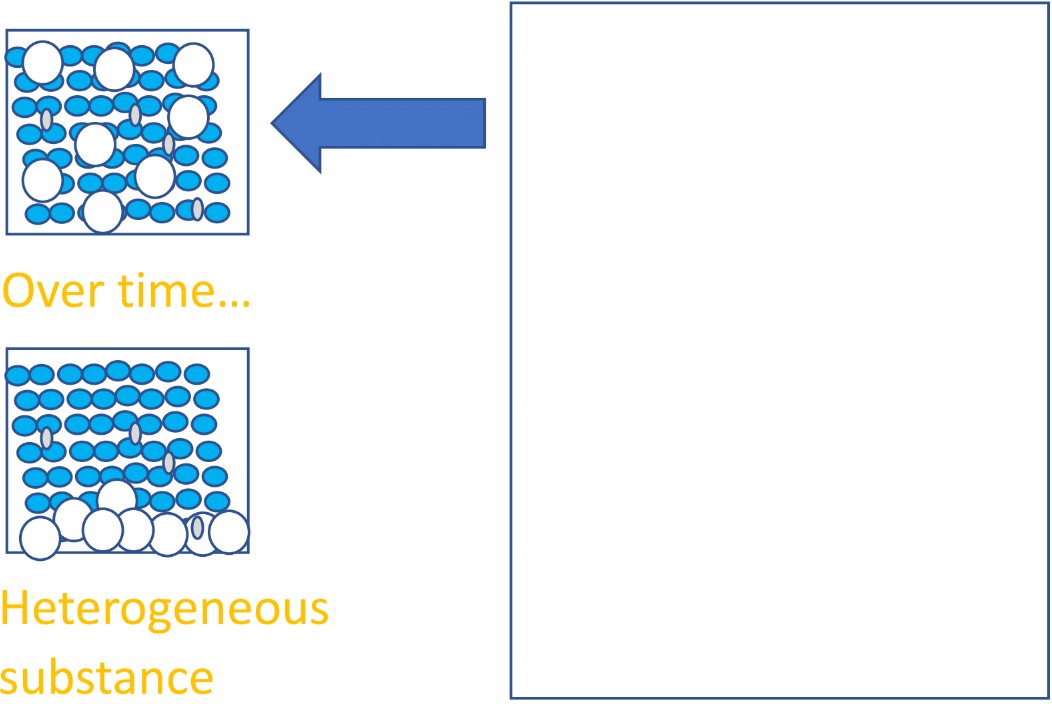
- A mixture is made up of two or more chemically distinct substances that are not chemically combined. For example, a glass of orange juice or milk. Milk is not made up of “milk molecules”, milk is a combination of water, fat molecules, sugars and proteins. These parts are not chemically joined together, they are distinct molecules with their own properties that are just physically mixed together.
This means that the substance might not be uniform, or evenly mixed throughout. Take a small sample of it and it might not have the same composition as another sample. You see this on supermarket shelves: orange juice separates over time because denser parts like the orange bits sink to the bottom of the container, leaving the clearer watery part near the top.
See below for an image of milk as an example of a mixture and a heterogeneous substance. The substance is a physical combination of several different chemicals; water is one of them.