In this lesson, we will learn:
- To recall the equilibrium ionization expressions for weak acids and bases.
- How to relate Ka and Kb for conjugate acid/base pairs.
- How to calculate concentration of aqueous ions in weak acid/base solutions.
Notes:
- In Autoionization of water, we looked at the equilibrium:
2 H2O(l) H3O+ (aq) + OH- (aq)
The equilibrium constant was expressed as:Kw = [H3O+] [OH-] = 1 * 10 -14 at 25oC
We saw the effect of adding strong acids/bases to equilibrium concentrations of water and dissolved ions. That was straightforward because strong acids and bases experience 100% dissociation in water.
We also saw in - In strong and weak acids and bases that weak acids and bases do not experience 100% dissociation. This makes expressions for their dissociation in water more complicated.
- Every weak acid has an acid dissociation constant, Ka and a weak base a base dissociation constant, Kb. These are equilibrium constants showing how much the acid/base dissociates when dissolved in water (aqueous solution).
For a weak acid HX dissolved in water:Ka =
For a weak base B dissolved in water:Kb =
- Just like in the ionization of water and other equilibria; this is an equilibrium constant expression. This means that the higher the Ka/Kb value, the greater the degree of dissociation (because the concentrations of the dissociated ions, in the numerator, are larger values) and therefore the stronger the acid or base.
- Remember that for strong acids and bases Ka/Kb values are not normally used. This is because in the Ka expression, their [HX] or [B] is equal to or almost zero due to complete dissociation, so the values are incredibly large.
- For strong acids, pKa is used instead of Ka. pKa is the negative logarithm of the Ka value and is more appropriate to use for strong acids, instead of the extremely large Ka values they have.
- In Conjugate acids and bases, we learned in a conjugate pair that a stronger conjugate acid will have a weaker the conjugate base. This relationship affects the concentration of aqueous ions and therefore affects the Ka and Kb values for a conjugate acid/base pair!
Consider the equations for the conjugate pair acid-base pair HX and X-:Conjugate acid: HX + H2O X- + H3O+ Ka = Conjugate base: X- + H2O HX + OH- Kb =
- Both equations depend on [X-] and [HX] so Ka and Kb themselves can be related, and terms cancelled out:
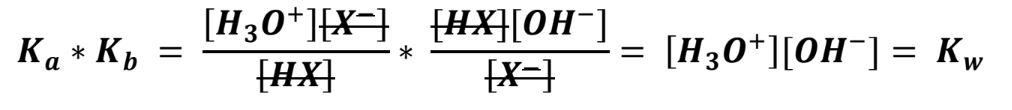
As you can see, the result is the product of [H3O+] and [OH-] which is the expression for Kw. Therefore for a conjugate pair:Ka * Kb = Kw
- Both equations depend on [X-] and [HX] so Ka and Kb themselves can be related, and terms cancelled out:






