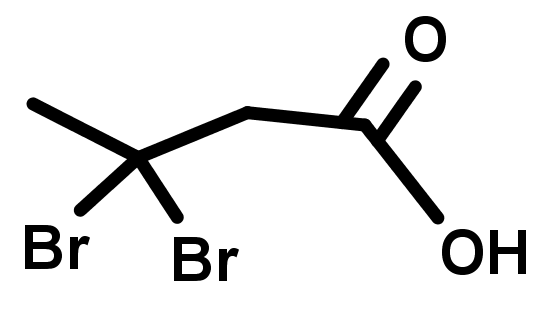
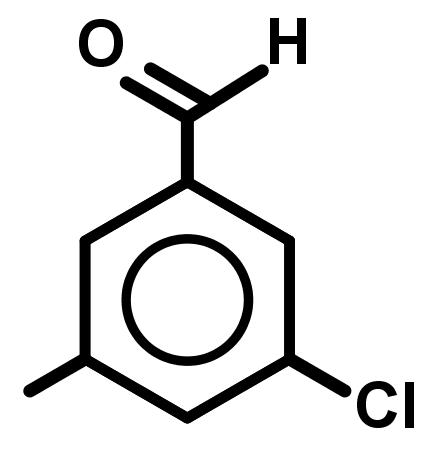
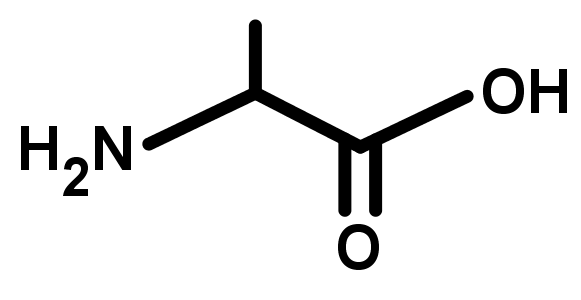
- The names and structures of the major organic functional groups.
- The naming priority of the major functional groups in organic chemistry.
- How to apply IUPAC systematic naming and priorities to more complex organic compounds.
Notes:
- There is a huge number of organic functional groups, but there are around 10 that you will see far more than any others. The most common are acylic groups containing O and N with single or double bonds to carbon.
- Dont worry about naming compounds with new functional groups. The IUPAC systematic nomenclature is systematic, which means whatever molecule you have, you follow the same rules to name it. Weve seen these rules before:
- Identify the longest continuous carbon chain. This is the parent chain of the compound
- Identify the functional groups in the molecule. Find the highest priority functional group and use the suffix for this group.
- Number the carbon chain, starting at the chain end which results in the lowest combination of numbers for the substituents.
- This means, for example, 2-methyl-3-octene is correct instead of 6-methyl-5-octene.
- Give the highest priority functional group the suffix of the compound name.
- The groups shown in this lesson are listed in order of decreasing priority. Notice that higher priority groups generally have multiple bonds to oxygen and nitrogen.
- All other functional groups can be numbered and named in alphabetical order once the highest priority group has the suffix and the carbon chain is numbered.
- Naming aromatic compounds is not clear-cut. There is no ruleset that will always find the best name for an aromatic compound because the old pre-IUPAC names (which are not systematic) are still in use and often preferred to the new IUPAC name.
However, the following is a sensible ruleset that will lead you to a recognized name for aromatic organic compounds: - Treat benzene rings as a 6-carbon alkene group.
- Treat groups below alkenes as lower priority. Alkynes, alkanes, ethers, halogens and nitro groups. With just these, the suffix will be benzene.
- Treat any carbon chain longer than 6 carbons and groups higher than alkenes as higher priority to the benzene ring it will take the prefix phenyl- here.
- The following functional groups are listed according to their priority, highest first, in the naming of organic compounds:
Key for the table:
R, R', R'' = can be any alkyl or aromatic group.
X = halogen (F, Cl, Br, I)