In this lesson, we will learn:
- To recall the definition of a buffer solution and its components.
- How to predict the shift in equilibrium when acid or base is added to a buffer solution.
- How to calculate the pH of a buffer after the addition of strong acid or base.
- How to calculate the Ka of a weak acid from the buffer region of a titration.
- How to find the quantities needed to make buffer solutions of a given pH.
Notes:
- A buffer solution is a solution of a weak acid or base with its conjugate pair that can resist changes in pH when a small amount of strong acid or base is added.
Buffer solutions are made by mixing a weak acid or base with a large amount of its conjugate pair, even so that there is equal amounts of both. - Above all, in chemistry buffers are known as mixtures that resist chemical changes made to them. Outside of chemistry, a buffer is something that acts a barrier or shield to another action or object, like buffers on a train track that cushion trains against their own movement, making them stop when they arrive at a terminus station. In chemistry, a buffer solution cushions and halts a chemical change being made in a few ways.
- Buffers resist changes in pH by keeping H3O+ concentration nearly constant; it’s able to do this as the solution contains the conjugate acid/base AND its pair in equilibrium. This is seen with weak acids and weak bases.
- For example, a buffer solution of methanoic acid (HCOOH) and methanoate (HCOO-), its conjugate base. There is an equal amount of conjugate acid and base.
The weak acid is in equilibrium with its conjugate base (methanoate, HCOO-) in the equation:H2O + HCOOH(aq) HCOO-(aq) + H3O+(aq) - If a strong acid such as nitric acid is added, the extra H3O+ ions it produces will react with the methanoate (HCOO-) and produce more HCOOH – the reverse reaction above will occur.
HCOO-(aq) + H3O+(aq) → H2O + HCOOH(aq)
This removes the H3O+ that was originally added and returns the pH to near its original value. - If a strong base such as sodium hydroxide is added, the extra OH- ions will react with some H3O+ ions in solution to produce water.
OH-(aq) + H3O+(aq) → 2H2O(l)
This excess water will shift the equilibrium to the right, producing more HCOO- and H3O+ - the forward reaction in the equilibrium, which will return the pH back to near its original value.H2O + HCOOH(aq) → HCOO-(aq) + H3O+(aq)
Because it’s an equilibrium, we give this an equilibrium constant expression. This is just an acid dissociating into its conjugate base so it is a Ka expression: - If a strong acid such as nitric acid is added, the extra H3O+ ions it produces will react with the methanoate (HCOO-) and produce more HCOOH – the reverse reaction above will occur.
- For example, a buffer solution of methanoic acid (HCOOH) and methanoate (HCOO-), its conjugate base. There is an equal amount of conjugate acid and base.
- WORKED EXAMPLE:
- In a buffer made of 0.5 mol methanoic acid (HCOOH) and 0.5 mol methanoate (HCOO-) in 1L water, 0.1 mol H3O+ is added.
Look at the equilibrium again:H2O + HCOOH(aq) HCOO-(aq) + H3O+(aq)
With 0.1 mol H3O+ added, 0.1 mol HCOO- will react with this ‘disturbance’ to the equilibrium, which shifts to the left to oppose the change. This changes the equilibrium amounts of moles:
Moles of acid (HCOOH)
Moles of base (HCOO-)
Before
0.5
0.5
Change
+0.1
-0.1
After
0.6
0.4
This change to the acid and base quantities will affect [H3O+] in this buffer solution. When a buffer has equal amounts of conjugate acid and base, the terms cancel out and [H3O+] will be equal to Ka, which makes pH = pKa: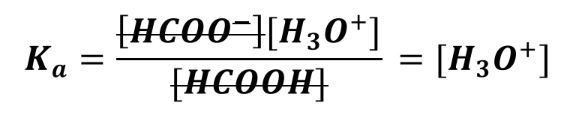
When the amounts are unequal, a constant will be in front of [H3O+].
In our example, after 0.1 mol of acid was added:
With these expressions, we can easily work out [H3O+] and then the pH of the buffer before and after the acid was added, using the Ka of the acid.1
With Ka (HCOOH) = 1.8*10-4, we work out the pH before the acid was added: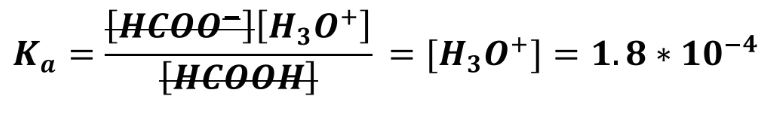
We re-arrange the Ka expression to solve for [H3O+] after the acid is added:→
This shows the effect of a buffer solution. 0.1 mol acid was added to the buffer, and the pH change was from 3.74 to 3.57, only 0.16 units. Consider that 0.1 mol H3O+ added to 1L pure water (pH 7) would give a pH of 1, a change of 6 units!
- Buffer solutions can resist changes in pH because of the equilibrium between the weak acid/base and its conjugate pair. The equilibrium will simply shift to remove or produce more H3O+ to oppose the change made when you added strong acid or base! See the diagram below, where a buffer is made with equal amounts of conjugate acid and base.
- In a buffer made of 0.5 mol methanoic acid (HCOOH) and 0.5 mol methanoate (HCOO-) in 1L water, 0.1 mol H3O+ is added.
- The pH of a buffer is also unaffected by dilution. This is unlike acids or bases on their own; diluting a strong acid solution tenfold will decrease H3O+ concentration tenfold, and increase pH by 1. However, Buffers do not change pH when diluted because the conjugate acid and conjugate base in them are diluted equally.
For example, take a buffer solution with equal amounts of methanoic acid, HCOOH, and methanoate, HCOO-. The Ka expression would be:
If the buffer was 1M HCOOH and 1M HCOO-, a tenfold dilution would decrease the concentration of both to 0.1M. If conjugate acid and base are equimolar, they will cancel out anyway: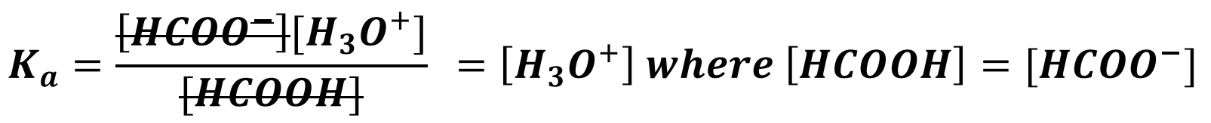
The above equation shows that in an equimolar buffer (where acid and base concentrations are equal), Ka is equal to H3O+ therefore pKa = pH.
Even in a buffer where acid and base concentration are not equal, dilution does not change the [acid]/[base] concentration ratio. For example, 1M acid with 0.6M base diluted tenfold will
Both of these scenarios the pH of buffers are unaffected by dilution because both acid and base are diluted equally! - The effect that adding strong base/acid to a weak acid/base buffer solution, when plotted on a curve, resembles a titration curve – the middle section where pH changes slowly is known as the ‘buffer region’ where the buffer equilibrium is established. The exact middle of this buffer region represents the pKa of the buffer, which is equal to the pH when there is a 1:1 conjugate acid:base ratio in the solution.
- The effect of a buffer is seen when a weak acid is being titrated by a strong base, in the initial part called the buffer region. See the image below:
During the beginning of the titration, while base is being added, there is only a very small increase in pH. This is because as the OH- base is being added, most of it is being reacted away by the H+ from the weak acid.
The weak acid’s Ka equilibrium, responding to a drop in [H+] now shifts to produce more H+, which will need to be reacted away by more OH- from the strong base!
To find the pH in a weak acid-strong base titration, chemists use the Henderson-Hasselbach equation:
This equation is very useful to find the Ka of an unknown weak acid using the pH1/2, which is the pH when half of the volume of base at equivalence point has been added.
At pH1/2, you have added half as much base as there originally was acid.
Because half of the acid has been neutralised, you now have equal amounts of base and acid in your mixture. This makes the Henderson-Hasselbach equation a lot simpler: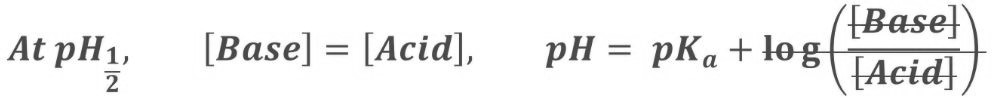
Therefore, at pH1/2:
Use the antilog (10-x) from this to find Ka. - The Henderson-Hasselbach equation can also be used to find the quantities needed to prepare buffers with a specific pH.
Using the equation, you can solve for a ratio of acid to base that is required for the buffer to give a specific pH.
This can be used for concentration, multiplied by your volume to find the number of moles you will need to originally add to create the buffer.






