In this lesson, we will learn:
magnitude of electric force, in newtons (N)
Coulomb's law constant,
magnitude of each charge, in coulombs (C)
distance between charges, in meters (m)
- Coulomb's law, which gives the electric force that one charged object exerts on another
- Calculating electric force for different arrangements of charges
- Electrostatics deals with electric charges that are at rest ("static")
- Charge is a scalar quantity. It can be positive or negative. The positive or negative character of a charge is called polarity.
- Like gravity, electric forces act at a distance. Unlike gravity, which always pulls objects together, electric forces can either push apart or pull together charges.
- Like charges (both positive or both negative) will repel each other
- Opposite charges (one positive and one negative) will attract each other.
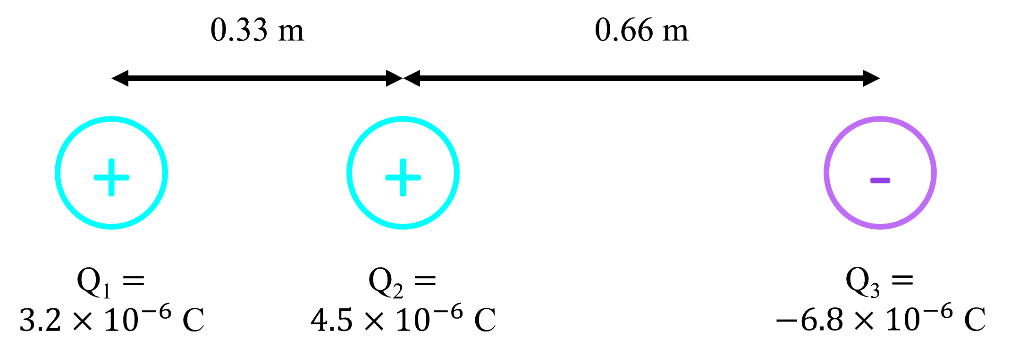
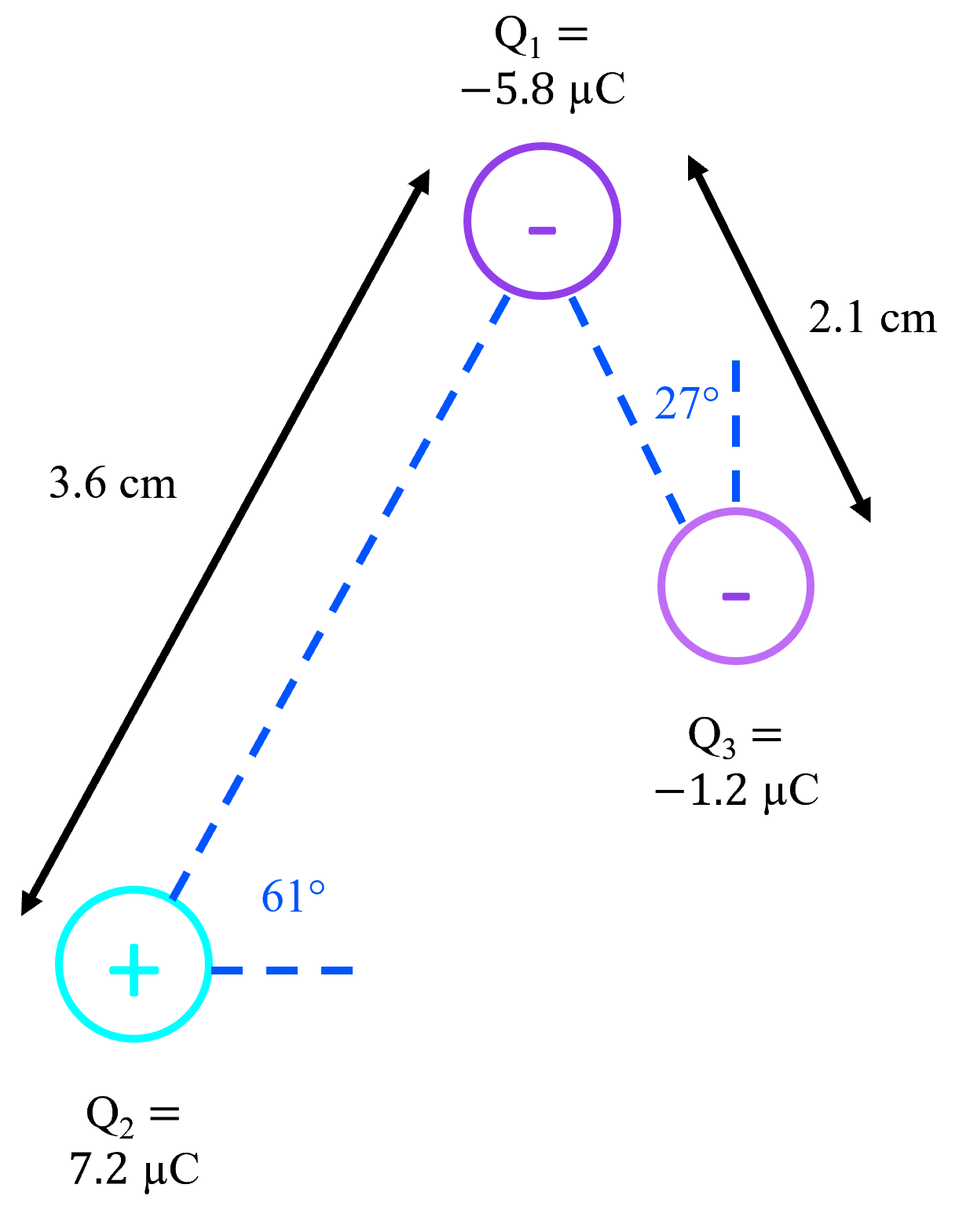
- Coulomb's law describes electric force (Fe).
- k (Coulomb's constant) is an experimentally determined constant that relates the size of the charges (Q1 and Q2) and radius (r¸ distance between charges) to the magnitude Fe.
- Coulomb's law only gives the magnitude of Fe and not the direction, indicated by the absolute value sign on |Fe|. Notice that k, Q, and are all scalars: there are no vectors on that side of the equation that could give Fe a direction. The direction of Fe must be found by considering if the charges involved would be attracted or repelled, based on their polarities.
Coulomb's Law (Electric Force)
magnitude of electric force, in newtons (N)
Coulomb's law constant,
magnitude of each charge, in coulombs (C)
distance between charges, in meters (m)