In this lesson, we will learn:
- To recall the definition of an indicator and their uses in chemistry.
- How to describe an indicator’s action in terms of equilibrium.
- How to use the Ka expression to predict an indicator’s color at a given pH.
Notes:
- In chemistry, measuring pH is done by using indicator solutions. There are many different indicators in chemistry but all of them are either a weak organic acid or base that is a different color to its conjugate pair. Some examples are:
Indicator
Acid (protonated) color
Base (deprotonated) color
pH range
Phenolphthalein
Colorless
Pink
8.2-12.0
Bromothymol blue
Yellow
Blue
6.0-7.6
Alizarin yellow
Yellow
Red
10.0-12.0
Each indicator has a specific pH range in which it will change color by accepting or donating a proton. The choice of indicator used in an experiment will depend on the solution being investigated and its pH. - As with any weak organic acid or base, you can write an equilibrium equation to show the change from acidic to basic form. Because indicators are quite complicated molecules, we either use abbreviations of the name in equilibrium equations (e.g. ‘Aliz’ for Alizarin) or simply In (for Indicator).
HIn + H2O H3O+ + In-
For example, with bromothymol blue which is yellow in acidic conditions and blue in basic conditions:HIn + H2O H3O+ + In-
Yellow Blue
In basic conditions, [H3O+] is low. The equilibrium will shift to the right to produce more H3O+ and as a consequence, more In-. This will eventually cause there to be more In- molecules than HIn molecules – more blue molecules than yellow molecules. This causes the color change from yellow to blue.
In acidic conditions, [H3O+] is high. The opposite effect happens here, where the equilibrium shifts left, reacting blue In- molecules to produce more yellow HIn molecules. This causes the color change from blue to yellow when conditions become more acidic. - As with any equilibrium, we can write an Keq equilibrium expression for this reaction. This equilibrium is just showing dissociation to give off H+ or accepting H+ to go to a conjugate base. This is acid dissociation! So we label it Ka:
Ka =
The point where the indicator is on the brink of changing color (or ‘halfway through’ changing color) is called the endpoint. This is where [HIn] = [In-]. At this point, the expression can be simplified: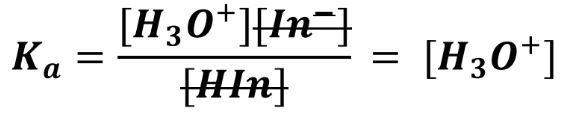
Therefore, the Ka of the indicator at the endpoint equals [H3O+]. This also means that at the endpoint, pH = pKa of the indicator. - Indicators can be added to different acids or bases to compare their relative strengths. For example: Methyl orange is an indicator which is red when protonated (when in acidic solution) and yellow when deprotonated (when in basic solution).
In- + HA A- + HIn
Yellow Red
Methyl orange is added to a solution of acid HA, where it turns yellow. It is also added to another acid HB where it turns red in solution.
If methyl orange is yellow (In-) in the presence of HA, it means that more of its molecules are deprotonated, and therefore that HA is in protonated form. Since a stronger acid (a stronger proton donor) will always protonate a weaker acid, this suggests that methyl orange is the stronger proton donor, and is therefore a stronger acid. Methyl orange is a stronger acid than HA.
Conversely, if methyl orange is red (HIn) in a solution of HB, it means that more of its molecules are protonated than deprotonated, and therefore HB is mostly deprotonated. This suggests HB is a stronger proton donor and therefore HB is a stronger acid than methyl orange.
The relative acid strength is therefore HB > HIn > HA






