- What is meant by the general term bonding and the categories of bonding.
- Why different chemical substances display different types of bonding.
- How different bonds and their varying strengths lead to different chemical properties.
- The link between the bonding, structure and properties of chemical substances.
Notes:
- There are less than 120 different types of atoms (the elements) in the periodic table, but there are tens of millions of different chemical species. How is this possible?
- Why atoms tend to combine into molecules in the first place. Why is it the case that most elements are just more stable joined to other atoms than existing as individual atoms? For a specific example, think of hydrogen existing as H2 molecules and not lone H atoms.
- Why bonding occurs in some species and not in others. Why does hydrogen exist as H2 but helium gas is not He2?
- Why some substances show completely different properties to others. Why does carbon dioxide have a low melting point and poor conductivity, while carbon, as graphite, has a high melting point and can conduct electricity?
- Bonding is a very general word. It can be used to describe any of the attractive forces that act between or inside molecules, and if you are asked to describe the bonding in a substance you should talk about any attractive forces present in the substance.
Before learning any types of bonding, recall the principles of electrostatic forces we saw in the Periodic Table and Elements chapter. They will help explain why different types of bonding exist: - #1: Oppositely charged particles attract each other, while particles of like charge repel each other.
- #2: The greater the charge difference of two particles, the greater their force of attraction (for example, the attractive force of a 2+ charge attracting a 2- charge is greater than the attractive force of a 1+ charge attracting a 1- charge).
- #3: Attractive forces between oppositely charge particles decrease with distance.
- #4: Repulsive forces between like charged particles decrease with distance.
- There are two broad categories of attractive forces (bonds) in chemical substances:
- Forces that hold the atoms of a molecule or compound together, acting between the atoms inside molecules, are intramolecular forces. All chemical bonds are intramolecular forces.
- Forces and interactions in between molecules are called intermolecular forces. These are the forces that often determine if something is a gas, liquid or solid at room temperature.
- Different substances (carbon monoxide and carbon dioxide) are made by rearranging atoms WITHIN a molecule. This would be different intramolecular forces.
- Different phases of a substance (carbon dioxide gas or liquid carbon dioxide) are made by overcoming the intermolecular forces BETWEEN molecules. This is to say:
- In a solid, the attractive forces holding the molecules together have not been overcome. This is why the particles are densely packed and have little space between them.
- In a liquid, the intermolecular attractive forces have been partially overcome. This is why substances in liquid phase can flow and are usually less dense than their solid phase.
- In a gas, the intermolecular forces have been completely overcome. This is why gases flow extremely easily and the particles, supplied with a lot of energy, are very energetic and gases take up a lot of volume compared to their liquid or solid phases.
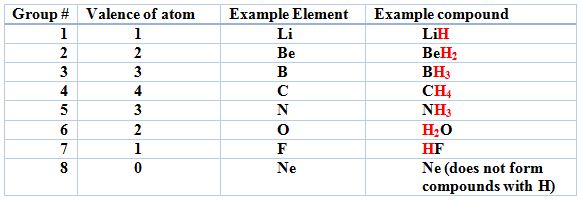
- How an atom bonds is mostly determined by the valence of the atom; this is the number of unpaired outer shell electrons. The valence practically tells you how many bonds the atom can make:
- In Electronic structure: 288 rule and Electronic structure: Subshells, we saw that the shape of the Periodic Table is made to show the different electron subshells or orbitals that atoms have the s, p, d block etc.
- To find the number of valence electrons in the outer shell, show the outer shell as four equal orbitals, not separate s and p sections. We will find out why later. Fill in the valence shell by adding single unpaired electrons to the four orbitals, then start pairing them up with the 5th onward.
- Because paired electrons generally dont bond, only the unpaired electrons are available to make bonds these are the valence electrons.
- Across groups 1-8 (ignoring the d-block), the valence of the groups are: 1, 2, 3s, 4, 3, 2, 1, 0. The higher the valence of an atom, the more bonds it can make. This comes from the outer shell electron configuration. Being comfortable with electron configurations will help your understanding of bonding a lot!
- The properties a chemical displays are due to the types of bonding and interactive forces between its atoms and molecules, and the types of bonding a chemical shows is because of its atoms' valence, and the electrons being able to make certain bonds in order to gain a full valence shell.
- Example 1: Neon is a noble gas with 8 valence electrons all paired up. Therefore neon atoms have a valence of zero, and they don't make bonds between atoms to form molecules or compounds.
- This means they don't have strong intramolecular forces; a sample of neon gas exists as millions of single atoms freely floating in space.
- Therefore they are entirely limited to weak intermolecular forces and so have very low boiling points, are gases at room temperature and cannot conduct heat or electricity.
- Example 2: Carbon atoms have four unpaired valence electrons, meaning carbon atoms have a valence of four and each atom can make four covalent bonds (a type of intramolecular force) with other atoms.
- Because each atom can 'connect' with a strong bond to four others, pure carbon is found in some forms that have the atoms in a single giant structured network, made of millions of individual carbon atoms all covalently bonded together. This is the structure of diamond.
- When doing this, because the structure is effectively one giant molecule and no one atom can be disturbed without disrupting the entire structure, diamond is the hardest substance known to man and, practically speaking, cannot be melted.
- The valence bonding and interactive forces that exist in a chemical species gives rise to the structure of the molecules it makes, which dictate its properties. Understanding the link between bonding that leads to structure that leads to properties is crucial and will allow you to make some predictions about certain chemical substances even when given just the formula.
In short, there is just an incredibly large number of different possible combinations and arrangements of atoms. The ways an atom can combine to form larger, more organized structures, and the attractive forces that make this happen is known as the bonding of the substance.
There are several detailed theories of bonding, which try to explain things like:
You have seen simple answers to some of these already such as in Electronic structure: 288 rule, where we learned that atoms like to have a full outer shell of electrons. We also learned about electronegativity as the ability to attract electrons and complete a full outer shell.
These are simple explanations which work well for now. To start, remember that valence (the number of outer shell electrons) and electronegativity heavily affect how atoms can bond.
This chapter will look at the different types of bonding we see in substances and how these lead to varying structures and properties (like melting point, electrical conductivity etc) we observe. We also look at how to predict when certain bonding will occur and how to represent it when drawing molecules.
Intramolecular forces (ionic and covalent bonds) are much stronger than intermolecular forces. When a substance melts or evaporates, it is the intermolecular forces being overcome, not the intramolecular forces!
It is important to understand the difference now: