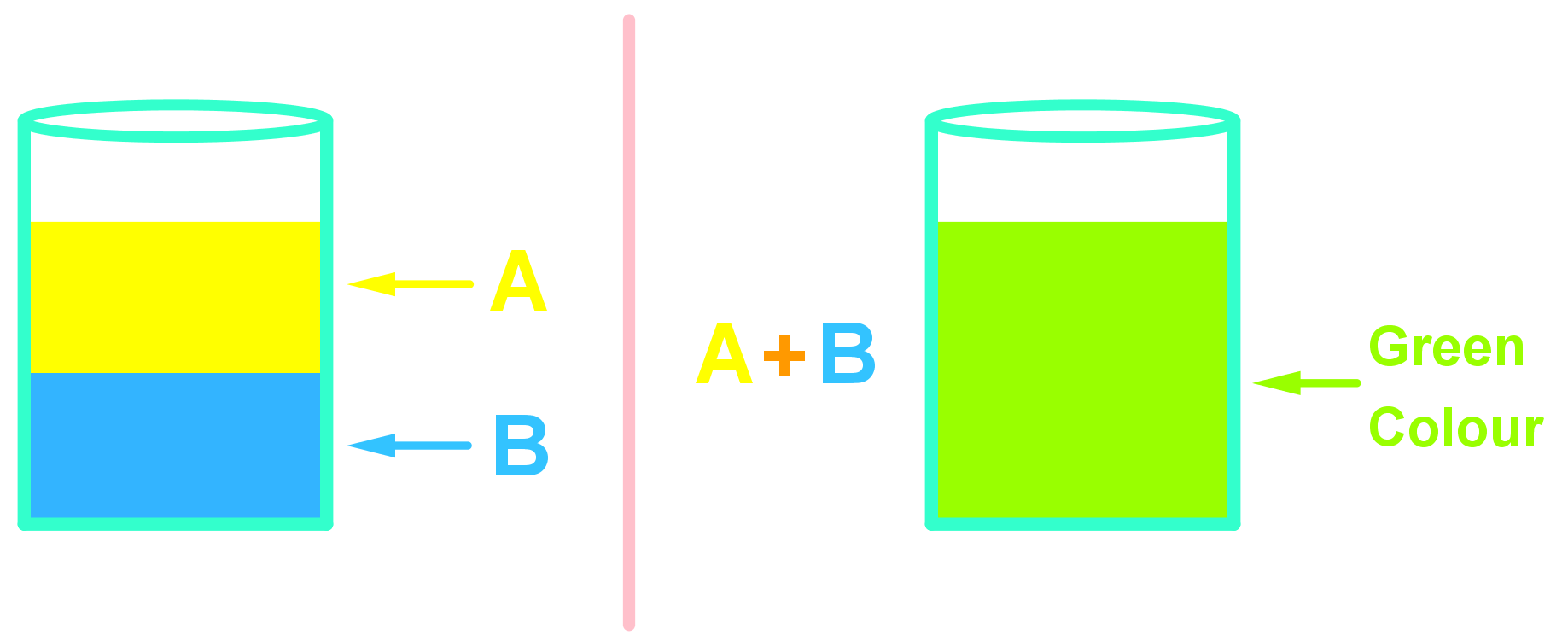- What a solution is and the definitions around it.
- Why most chemical reactions are done in solutions and why they are useful.
- The definitions of solubility and factors that affect it.
Notes:
- Solutions are important in chemistry because most chemical reactions and processes are done with the reactants dissolved in solutions. There are good reasons for this that will be revealed throughout this chapter.
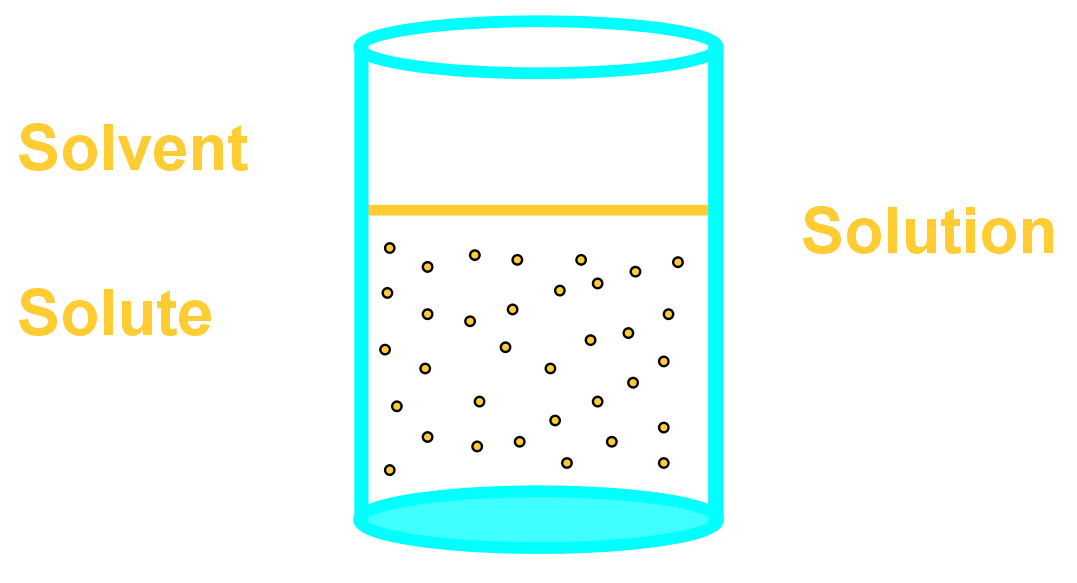
- A solution is a single-phase homogenous (evenly spread) mixture of two components called the solvent and the solute:
- The solvent is the chemical in larger amount – its greater number of molecules surround the dispersed, outnumbered solute. Chemists say that a solvent dissolves a solute.
- The solute is the chemical in smaller amount – its smaller quantity means individual molecules of solute are dispersed throughout the solvent molecules and surrounded by them. Chemists say a solute is dissolved by a solvent.
- The diagram below illustrates the definitions above. Remember a few key points below:
- The solvent is also made of molecules, only in much greater numbers than the solute, easily in 1:1000 ratio of solute to solvent, and normally larger than this!
- Solute particles are far too small to be visible when dissolved. Diagrams showing them visible are just for illustration!
- In a solution, the solute (e.g. chemical A) molecules are so outnumbered by the solvent (chemical B) molecules, that we can think of each individual chemical A molecule as fully exposed to the chemical B molecules and anything else that might be dissolved in chemical B as well – all particles are colliding with energy into one another.
- This is extremely useful for chemicals that are solids at room temperature! By dissolving them, we can get them to 'behave' like liquids without melting them, which might have needed very high temperatures. Then, reactions can be done with them.
- The solution 'state' allows atoms and molecules to collide with each other freely at mild conditions as if they were liquids. This is extremely useful in chemistry! Think about chemical A and chemical B reacting in the following situations:
- Chemicals A and B are both solids: Particles in solids are very close together and tightly packed. Very few particles of A and B will be making contact with each other at any one time and so the reaction is extremely slow.
- Chemicals A and B are both gases: Gas particles are very high in energy and will spread through space very quickly. The reactants will not collide with each other much and will escape the reaction vessel unless it is air tight.
- Chemicals A and B are both liquids: This is an advantage, but the two chemicals might not be able to mix together – they may be immiscible. If they are then the chemicals form two separate layers where A and B particles are generally not colliding with each other.
- Solubility is the amount of a substance that can dissolve in a specific amount of another substance at a specific temperature. Whether a chemical can dissolve in another chemical and make a solution is largely to do with intermolecular forces acting between the molecules. A well-known phrase to explain solubility is "like dissolves like." This means a chemical that is polar – one that has hydrogen bonding – will dissolve other polar molecules and other polar molecules will dissolve it.
- The solute used, and the quantity of solute
- The solvent used, and the quantity of solvent
- The temperature the solution is made at.
- As stated above, like dissolves like:
- If a compound doesn't dissolve in a solvent we say it is insoluble. For example, when a non-polar compound and a polar compound are mixed together, they will not dissolve the other because of the different intermolecular forces between their molecules.
- If a compound does dissolve in a solvent we say it is soluble in the solvent. For example, if two polar or two non-polar compounds were mixed together, their similar intermolecular forces would enable a solution to form.
- A solution is made in a way that the amount of solvent is much greater than the solute it is dissolving – if there is too much solute added or not enough solvent it becomes a saturated solution. Saturation, then, is when a solution has dissolved the maximum amount of solute possible.
- An unsaturated solution is a solution that is able to dissolve more solute – it is not saturated.
- A saturated solution is a solution that cannot dissolve any more solute if it was added – like a sponge saturated with water, it is saturated with solute! If your solution is saturated, you need to add more solvent before you can add any more solute.
- When chemicals are dissolved they often show different properties to their undissolved state. This is particularly true of ionic compounds which can conduct electricity only in solution!
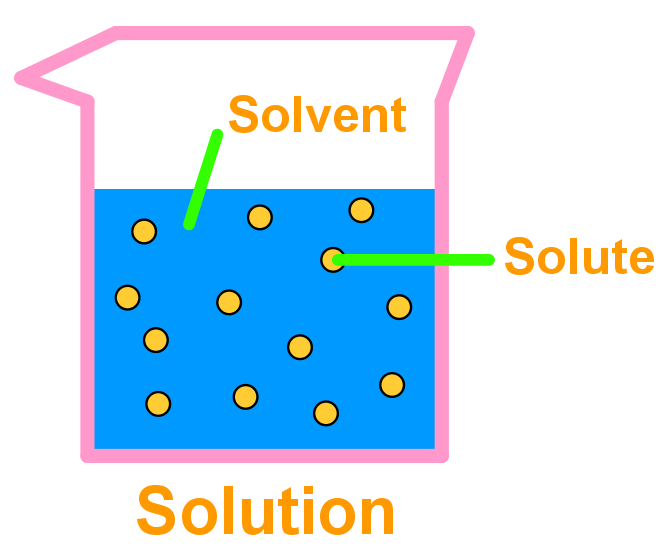
In the same way, less polar chemicals – those displaying just van der Waals intermolecular forces – will dissolve other non-polar chemicals and vice versa.
There are a number of factors that affect solubility and when you report solubility they must all be mentioned!